Accurate Detection Matters
cmAngio® An FDA-Cleared Artificial Intelligence (AI) based detection software to identify Breast Arterial Calcifications (BAC), an incidental finding in FFDM and DBT screening mammograms.
Results may be used for further patient management at the independent discretion of the interpreting physician.
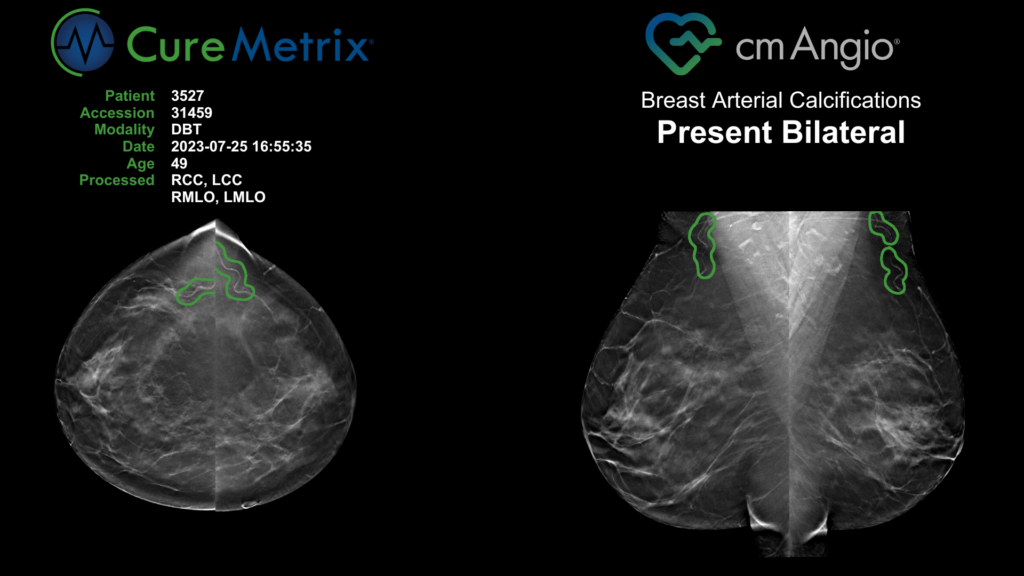
BREAST ARTERIAL CALCIFICATIONS
Breast Arterial Calcifications are medial calcifications of varying attenuation and length along the walls of the arteries and are currently considered a benign incidental finding in screening mammograms. Among women who have had screening mammograms, the prevalence of BAC varies from around 12 to 42.5%1. However, less than 5% of all instances of incidental findings of BAC in mammograms are reported2. There is no established guideline at a state or national level for identifying, evaluating, and reporting BAC, leading to inconsistent practices across different institutions and generally low rates of reporting.
cmAngio Key Benefits
- Provides visualization of BAC location on cmAngio scorecard so radiologists can quickly see what the algorithm has identified.
- Enables BAC identification and reporting without additional radiation for the patient or procedures by the radiologist.
- Allows radiologists to incorporate relevant information into their overall patient assessment and recommendations for follow-up.
- Helps identify women who have BAC and might otherwise have gone undiagnosed until other more serious symptoms became apparent.
cmAngio Key Features
SOURCES
1 CSBI https://csbi.ca/canadian-society-of-breast-imaging-position-statement-on-breast-arterial-calcification-reporting-on-mammography/
2 Internal CureMetrix analysis based on data collected from 2008-2018
*Please refer to the Operator’s Manual for additional details
*Disclaimer: cmAngio® is FDA-cleared for commercial use in the U.S. FDA 510(k) Submission #K232367